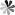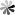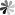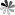RNAi in Gene Silencing
RNA interference (RNAi) and post-transcriptional gene silencing (PTGS) are conserved biological responses to double-stranded RNA that mediate resistance to endogenous parasitic and exogenous pathogenic nucleic acids and regulate the expression of protein-coding genes. endogenous triggers of the RNAi pathway include exogenous DNA or double-stranded RNA (dsRNA) of viral origin, aberrant transcripts of repetitive sequences in the genome ( For example, transposons) and pre-microRNA (miRNA). the mechanism of RNAi can be divided into two main steps: siRNA synthesis and RISC-mediated degradation or repression of translation of the target RNA. In RNAi, long double-stranded RNAs are cleaved into shorter small interfering RNAs (siRNAs) by the action of a nuclease (Dicer) enzyme. Each siRNA consists of 21 to 23 base pairs with two terminal unpaired ends. siRNA binds to RISC (RNA-induced silencing complex), resulting in RISC binding to the target mRNA and mediating its degradation or repressing its translation. siRNA can be used for functional genomics studies and to develop viral infections, neurological diseases and cancer (in vivo gene inactivation).
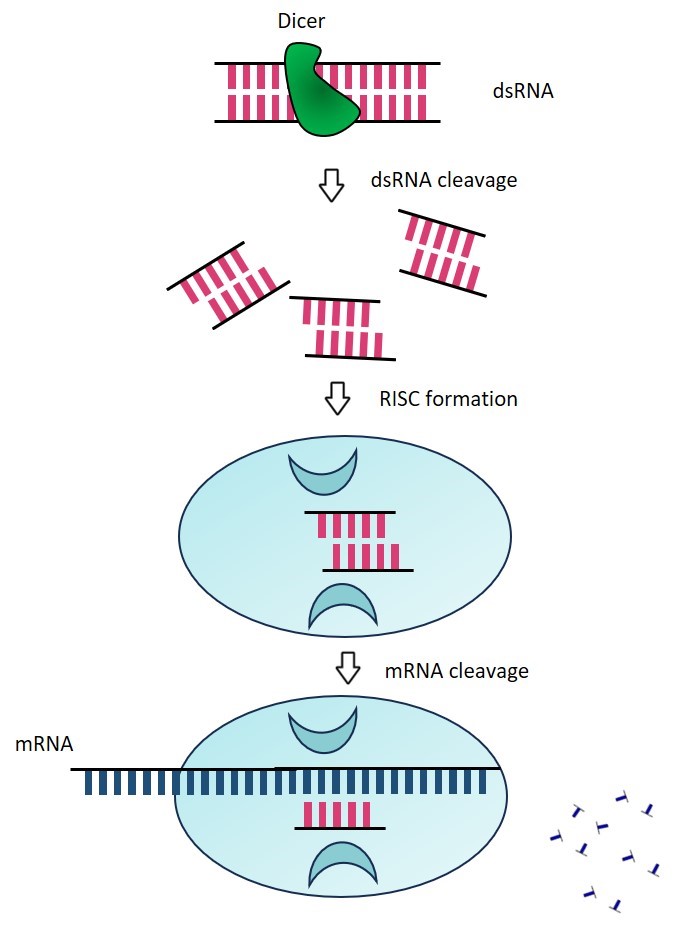 Figure 1. RNA interferes with the process of silencing genes.
Figure 1. RNA interferes with the process of silencing genes.
PseudoUridine Modifications in RNAi
- The RNA interference process involves important RNA molecules
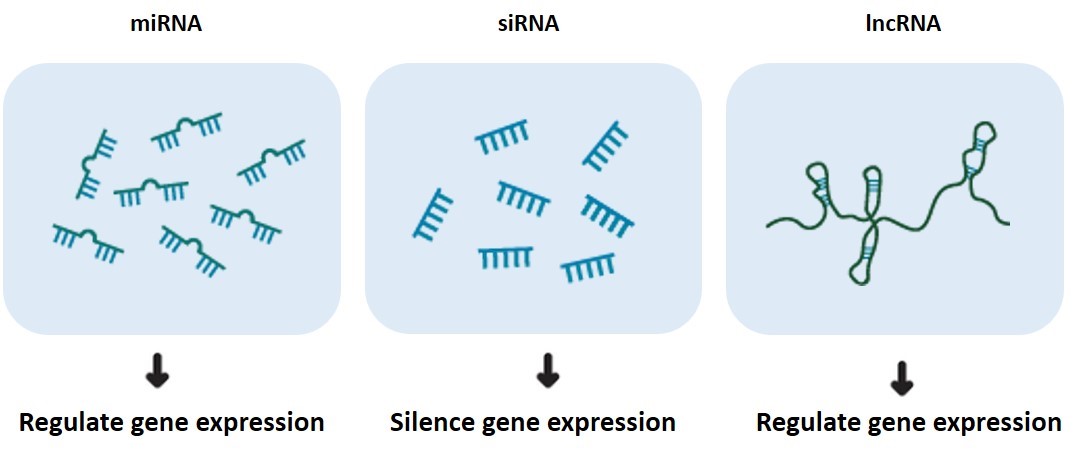 Figure 2. RNA molecules involved in the RNAi process.
Figure 2. RNA molecules involved in the RNAi process.
Long double-stranded RNA (dsRNA) longer than 30 bp are key activators of the innate immune response against viral infections and are the starting point for RNAi.
Small interfering RNA(siRNA) is a key molecule derived from dsRNA cut into double-stranded RNA of 21-25 bp size by RNase III (e.g. Dicer) in the cell and used to mediate gene silencing.
MicroRNAs (miRNAs) are produced by the action of Dicer enzymes to produce mature miRNAs that bind to RISC and participate in the regulation of gene expression.
RNA-induced silencing complex (RISC) is a complex formed by the complex of siRNA, Argonaute protein and Dicer enzyme. In RNAi, RISC uses the antisense strand of siRNA to cleave the target mRNA and achieve gene silencing.
- Biological Function of PseudoUridine Modifications in RNAi
- Stability enhancement: During siRNA synthesis, the introduction of pseudouridine modifications enhances siRNA resistance to nucleases to improve stability and regulatory accuracy.
- Enhanced RNAi efficiency: The properties of the pseudouridine modified siRNA surface are changed to the extent that the affinity with target genes is improved. As a result, siRNAs with pseudouridine residues exhibit enhanced and more precise target gene knockdown effects for more effective gene silencing.
- Reduced off-target effects: Since the affinity and binding specificity of pseudouridine modified siRNA and target genes are significantly improved, most of the off-target effects can be avoided.
- Improved safety: The pseudouridine modified siRNAs exhibit greatly reduced immunogenicity, liberating the restricted therapeutic potential and enabling safer and more efficient silencing of target genes.
PseudoUridine modification in RNAi plays an important role in enhancing siRNA stability, improving target gene knockdown efficiency, reducing off-target effects and minimizing immunostimulatory responses. PseudoUridine modifications have significant implications for the development of more effective and specific RNAi-based gene silencing therapies, and further provide more new ideas for gene therapy.