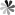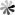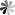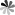PseudoUridine phosphoramidite for RNA synthesis
Phosphoramidites serve as building blocks in the chemical synthesis of oligonucleotides. In general, both DNA or RNA oligonucleotides are commonly synthesized by phosphoramidites through 3′→5′ direction. PseudoUridine phosphoramidite can help introduce pseudoUridine into RNA oligonucleotide. The usefulness of the PseudoUridine phosphoramidite allows for efficient and reliable syntheses of RNAs containing single or multiple PseudoUridine modifications. PseudoUridine modifications exhibit a range of effects on RNA stability and structure, depending on their locations. The biochemical roles of PseudoUridine have been found to affect the translation in tRNA and rRNA and the function of snRNA in splicing, and the PseudoUridine modified mRNA exhibit promising therapeutic potentials for gene replacement and vaccination.
PseudoUridine-3′-phosphoramidite
For RNA synthesis, the 2′-hydroxyl group of ribose in RNA phosphoramidites needs to be retained throughout the entire procedures of the RNA oligonucleotides synthesis and removed completely after the RNA synthesis. Tert-butyldimethylsilyl (TBS) is the most commonly used protection group for 2′-hydroxyl group of ribose. Due to the synthesis of RNA involved phosphoramidite is carried out via 3′→5′ direction, the 5’-hydroxyl group in ribose of pseudoUridine phosphoramidite needs to be protected and 4,4′-dimethoxytriphenylmethyl (DMT) is generally used as a protecting group. It has also been reported in the literature that the use of benzhydryloxy-bis(trimethylsilyloxy)silyl group (BzH) as protecting group for 5’-hydroxyl of pseudoUridine, followed by reaction with methyl tetraisopropylphosphorodiamidite to obtain pseudoUridine phosphoramidite.
However, there are some limitations for RNA synthesis involving phosphoramidites such as pseudoUridine phosphoramidite that TBS group is not completely stable under the basic conditions, and loss of the TBS group can lead to phosphodiester chain cleavage and 3′- to 2′-phosphate migration. In addition, RNA phosphoramidites need to be handled carefully to avoid degradation by exposure to RNase enzymes. Because RNases are ubiquitous, sterile conditions are necessary when handling RNA.
2'-deoxy-pseudoUridine 3'-phosphoramidite
The 2'-deoxy-pseudoUridine-3'-phosphoramidite belong to DNA phsphoramidites is used for the synthesis of DNA oligonucleotides. Hydroxy group on the 2’-position of pseudoUridine-3'-phosphoramidite is removed to produce the building block of oligodeoxynucleotides. It has been shown that oligodeoxynucleotides containing pseudoUridine can inhibit uracil-DNA glycosylase reaction. Furthermore, 2'-deoxy-pseudoUridine-3'-phosphoramidites support the incorporation of pseudoUridine derivatives into triplex forming oligodeoxynucleotides (TFOs). TFOs, also known as triple-stranded DNA, have an effect on preventing gene transcription. A study reported that the introduction of modified nucleosides such as pseudoUridine derivatives into TFOs can improve the stabilization of triple-stranded DNA.


-2-((bis(4-methoxyphenyl)(phenyl)methoxy).gif)