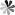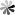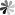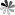Phosphate pseudoUridine is usually produced by the phosphorylation occurring in the 5’-position hydroxyl group of ribose of pseudoUridine. Phosphate pseudoUridine belongs to the C-nucleotides, in which the uracil moiety is combined with the sugar through its carbon atom rather than nitrogen atom forming a C-C glycosidic bond. The phosphate pseudoUridine can be divided in pseudoUridine 5ʹ-monophosphate (ΨMP), pseudoUridine 5ʹ-diphosphate (ΨDP) and pseudoUridine 5ʹ-triphosphate (ΨTP) depending on the number of linked phosphates.
PseudoUridine 5ʹ-monophosphate (ΨMP)
PseudoUridine 5ʹ-monophosphate is an ester of phosphate and nucleoside and consists of a phosphate functional group, a pentose ribose and a base uridine. In theory, ΨMP can be obtained by twice hydrolysis of pseudoUridine 5’-triphosphate, or through pseudoUridine coupled with one phosphate. A study has corroborated a metabolic process of pseudoUridine, including the single phosphorylation of pseudoUridine to afford ΨMP catalyzed by pseudoUridine kinase, then the C-C glycosidic bond cleavage to produce uracil and ribose 5’-phosphate mediated by ΨMP glycosidase. However, the metabolism of PseudoUridine in eukaryotes has not been thoroughly studied. And the functions of ΨMP are also not supported by sufficient literature. Interestingly, one recent study showed that ΨMP is toxic to Arabidopsis thaliana, and ΨMP may cause delayed germination and growth inhibition of this plant.
PseudoUridine 5ʹ-diphosphate (ΨDP)
PseudoUridine 5ʹ-diphosphate consists of two phosphate functional groups, a pentose ribose and a base uridine. The synthesis of ΨDP was reported as early as 1963, but to our knowledge, the biological applications of ΨDP are still to be discovered. The synthesis of PseudoUridine 5’-diphosphate has been achieved from PseudoUridine using the cyanoethylphosphate method to prepare the monophosphate and the nucleoside phosphoramidate method to make the diphosphate. During the synthesis, two side reactions occurred. One of these was an alkylation of the pyrimidine ring, the other involved an anomerization of the C-C glycosyl bond to give a steric isomer of ΨDP.
PseudoUridine 5ʹ-triphosphate (ΨTP)
PseudoUridine 5ʹ-triphosphate consists of three phosphate functional groups, a pentose ribose and a base uridine. It is well known that nucleoside triphosphates (NTPs) are served as the building blocks for the synthesis of RNA molecules whether in vitro or in vivo, both mediated by the polymerase. In addition, the chemical synthesis of ΨTP can be achieved by the reaction of ΨMP with tributylammonium pyrophosphate under basic conditions. In recent years, the applications of mRNA in the field of cancer immunotherapy and other biomedical fields have received increasing attention from researchers. However, the biological therapy of mRNA is limited by the immune toxicity in the host. This issue has been improved via mRNA modification that utilizes the naturally occurring modified nucleotides, such as pseudoUridine-5’-triphosphate. This pseudoUridine modified mRNA can serves as a promising tool for gene replacement and vaccination. For mRNA modification, successful molecular biology applications depend heavily on the quality and purity of NTPs. The simple and efficient synthesis of ΨTP to afford a high purity product can support the modification of RNA.