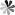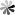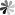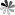2-ThiopseudoUridine (CAS 59464-18-5)
Triple-stranded DNA
Triple-stranded DNA, also known as H-DNA or Triplex-DNA, is referred DNA containing a triple helix structure with three intertwined oligodeoxynucleotides. Of these, the third strand binds to DNA double helix through the formation of Hoogsteen base pairs or reversed Hoogsteen hydrogen bonds. Oligodeoxynucleotides can bind to double-helical double-stranded DNA specific sequences to form triple-stranded DNA to prevent gene transcription or DNA replication, and this oligodeoxynucleotides is known as triplex-forming oligodeoxynucleotides (TFOs). The triple-stranded DNA technology is referred to as antigene technology.
Stabilization of Triplex-DNA via modified nucleoside
Using the antigene strategy, a large number of modified nucleosides have been synthesized to enhance the thermal stability of DNA triplexes formed by hybridization of the third DNA strands with DNA duplexes. Several studies showed that the use of homopyrimidine-oligodeoxynucleotides containing cytosine or 5-methylcytosine bases as TFOs under weakly acidic conditions resulted in significant stabilization of the resulting parallel triplex structures. This was due to the formation of protonated cytosine or 5-methylcytosine bases that could bind to guanine bases at the Hoogsteen base-pairing site. However, those acidic conditions limit the sequences of TFOs, therefore, antigene therapy using this strategy is not generally applicable.
To overcome this limitation, several modified nucleosides have been developed to mimic the structure of the 3-N-protonated cytosine base. 2'-O-Methylpseudoisocytidine is known to form a triplet base pair with a G-C base pair under neutral conditions, but TFOs containing this base could not stabilize the triplex structure sufficiently at neutral pH. 2'-O-methyl-2-thiouridine or 2-thiothymidine were reported that they can form quite stable parallel triplex structures which enhanced the thermal stability of triplexes likely through the strong stacking interaction of the 2-thiocarbonyl group with the 5'-upstream or 3'-downstream bases. Similarly, 4-thiopseudoisocytidine and 4-thiopseudoUridine containing thiocarbonyl group were found to stabilize the triplex formation.
Synthesis of 4-thiopseudoUridine (s4Ψ)
There has been reported a convenient method for the synthesis of 4-thiopseudoUridine (s4Ψ). Based on the analysis of 1H NMR spectral for this modified nucleoside, s4Ψ is supposed to prefer C3'-endo ribose puckering whose conformational properties are favorable for the stabilization of triplex formation. In the synthesis strategy, hydroxyl groups of pseudoUridine are protected to form 2',3',5'-tri-O-acetylpseudoUridine in first, and further treatment with excess POCl3 to give 2,4-dichloropseudoUridine derivative. Subsequently, the regioselectivity and specificity of the necleophilic reaction of 2-(trimethylsilyl)ethanethiol with 2,4-dichloropseudoUridine in the presence of triethylamine only generate the 4-thiolated compound. Then, 4-(2-trimethylsilyl)ethyl-4-thiopseudoUridine is formed via hydrolysis reaction of the 2-chloro-4-thiolated pseudoUridine derivative under base condition. Finally, the TBAF-mediated deprotection is occurred to form 4-thiopseudoUridine (s4Ψ).
Online Inquiry
Chemical Structure